Target general audience: an 8th grade-level audience (possibly even actual 8th graders!)
Reading time: 5 minutes
Jessica Desamero, PhD
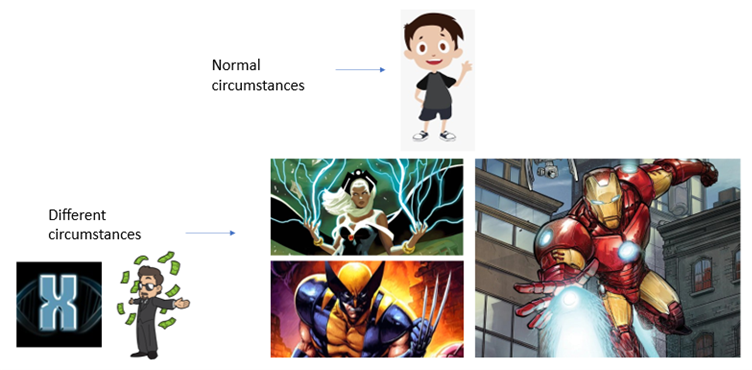
Superheroes are Extraordinary
In a world of fiction, superheroes are extraordinary beings. And depending on how they came to be, superheroes can have a wide range of powers and abilities. For instance, people born with a special X gene can control the weather or have razor-sharp claws. Genius billionaires can create expensive suits that allow them to fly and shoot lasers (Figure 1).
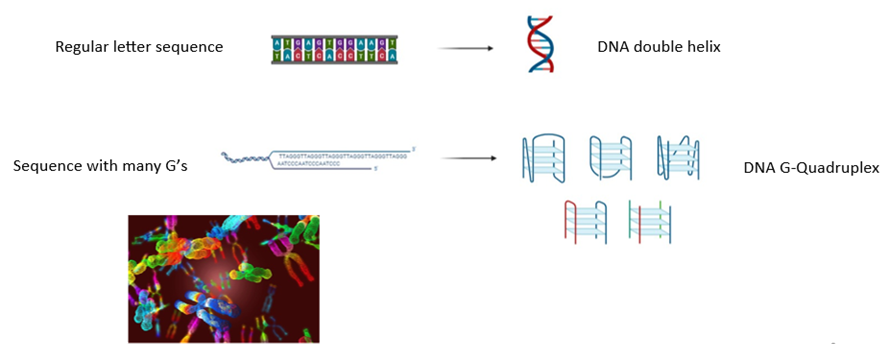
DNA G-Quadruplexes are also Extraordinary
DNA G-quadruplex structures are just as extraordinary as superheroes.
In cells, DNA sequences are made of A, T, C, and G bases, and they fold into organized structures. With a regular letter sequence, the DNA folds into its usual twisted ladder shape, or double helix. But in a sequence that contains many G’s, some of these G bases may come together to form special DNA structures called DNA G-quadruplexes, or qDNA. And depending on the cell’s environment, various quadruplex shapes are possible (Figure 2).
Depending on where they are, DNA G-quadruplexes can help manage some of the cell’s biological activities. They may even help stop some diseases from progressing. For example, quadruplexes that are found at the ends of chromosomes can play a role in cell death and cancer.
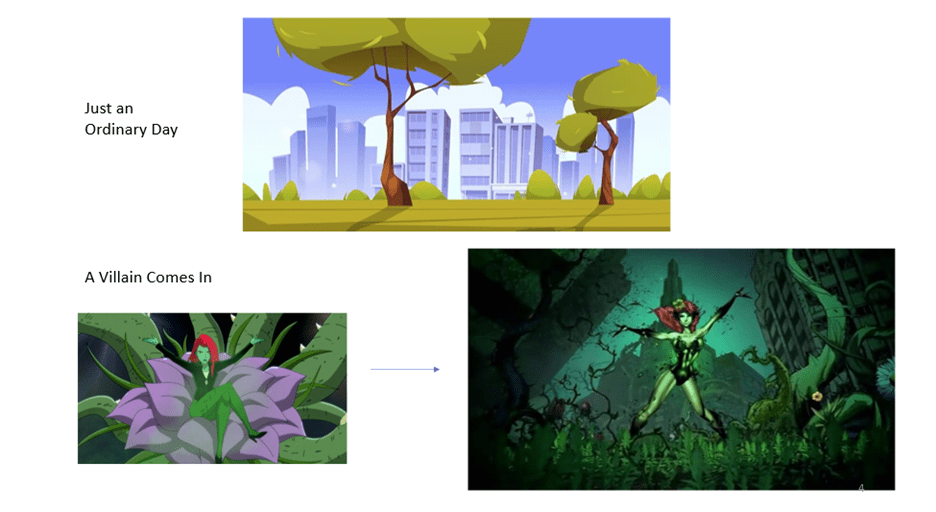
Superheroes Save the Day
Let’s say it’s an ordinary day in Gotham City. The sun is shining, and you’re admiring the native plants. Suddenly, the villain Poison Ivy shows up. She has the power to manipulate plant life and makes all the plants grow out of control. These plants then become vicious and attack the city, causing disaster. Enter the hero Batman to come save the day! With the help of his cool gadgets, he fights and defeats Poison Ivy, and eventually the city goes back to normal (Figure 3).

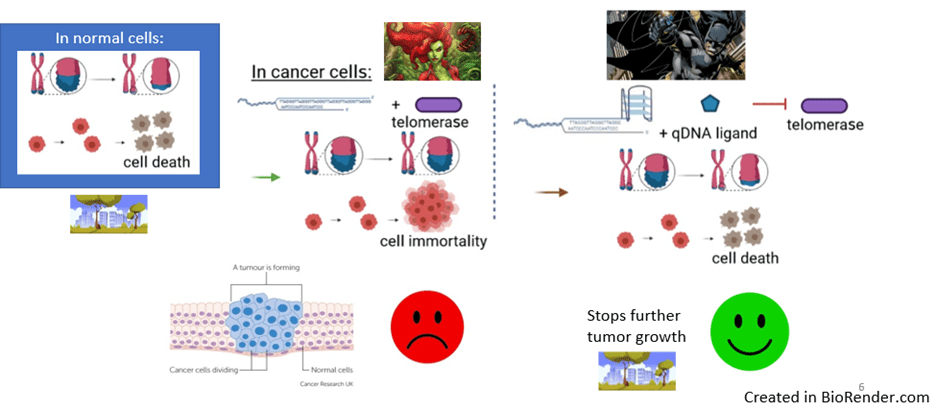
Quadruplex Heroes Stop Telomerase Villains
Now let’s look at non-reproductive cells. In a typical life cycle, cells grow and divide, age, and eventually die. As cells divide, the ends of chromosomes keep getting shorter until they reach a length that triggers cell death. But sometimes, a molecule called telomerase can get activated. This active telomerase would help keep the ends of chromosomes long. Then, since these ends won’t get shorter, cells will keep growing out of control, essentially becoming immortal. And with all these extra cells, tumor masses tend to form. This spells disaster, as tumors are trademarks of cancer.
Quadruplexes that form at the chromosomes’ ends offer a symbol of hope. With the help of qDNA ligands to keep them stable, these quadruplex heroes can stop telomerase villains from being able to interact with the DNA. This allows the chromosome ends to once again get shorter, restoring the signal for cell death and stopping further tumor growth (Figure 4). Thus, the body is saved!
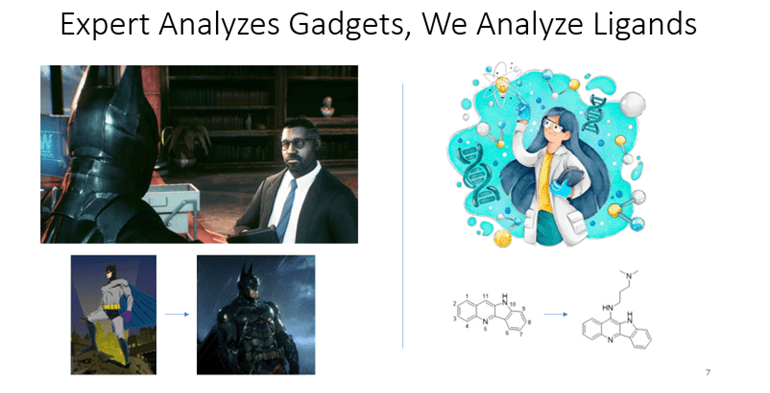
Expert Analyzes Gadgets & We Analyze Ligands
Lucius Fox, a genius technology expert, constantly thinks about how to improve Batman’s suits and gadgets. He then makes advanced upgrades to these suits and gadgets. These new and improved tools would help Batman fight crime more efficiently.
In our lab, we are biochemistry experts that want to better understand how ligands interact with DNA G-quadruplexes. We do this by analyzing how and where qDNA ligands exactly bind (Figure 5). This information could be useful when developing ligands as cancer drugs to make qDNA more stable.
More On the Origins and Purpose of Our Research
Our research focused on one ligand, NMM. The study involved analyzing the molecular properties of NMM and qDNA. One property we observed was fluorescence, or the ability of a substance to emit visible light after absorbing radiation that is not typically visible.
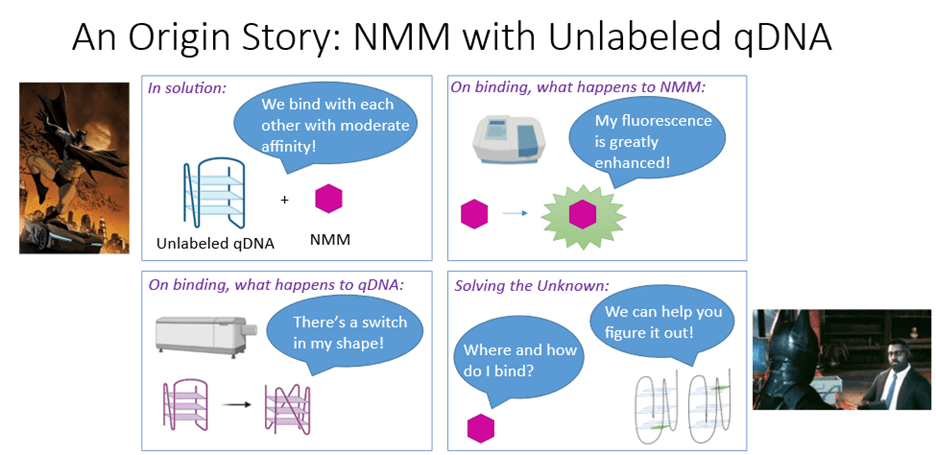
What is currently known about NMM interacting with qDNA (Figure 6):
- In solution, NMM binds to qDNA with moderate affinity, meaning that the interaction strength between NMM and qDNA is medium.
- By itself, NMM has weak fluorescence. But binding to quadruplex enhances this ligand’s fluorescence.
- Upon binding to NMM, quadruplex structure switches in shape, meaning it goes from one structural form to another.
What is still unknown (Figure 6):
Where and how does NMM ligand exactly bind to quadruplex? We know that NMM does interact with quadruplex, but we do not yet know the details behind these interactions.
Our research methodology:
To figure this out, we used several quadruplex sequences that each contain a light-up tag at a different position on the qDNA. This would allow us to assess the sections of the quadruplex one at a time.
Then we put each of these qDNA sequences and NMM ligand together in solution. After some time, we analyzed our samples and observed what happens, using multiple techniques that involve detecting light in different ways. With these tools, we were able to answer our main question.
Ultimately, We Want to Help Fight Cancer
qDNA ligands can serve as potential chemotherapy agents. This is why we want to know more about how ligands interact with quadruplexes, especially in a cell-like environment. By doing so, we can help in developing drugs to target and stabilize quadruplexes more effectively. Ultimately, this will aid in the fight against cancer.
Figure Sources
Header Image: Created by author in Microsoft PowerPoint
Figures 1, 2, 3, 5: Created by author in Powerpoint
Figures 4 and 6: Created by author, using a combination of Powerpoint and Biorender
This PhD research was done in the lab of Dr. Lesley Davenport at the City University of New York, Brooklyn College.
References
Desamero, J. (2023). Characterization of the Conformational Binding of N-methyl Mesoporphyrin IX with DNA Model Telomeric G-Quadruplex Forming Sequences. CUNY Academic Works. https://academicworks.cuny.edu/gc_etds/5543/
Leave a comment